
Compressed
Air Work in a Kerosene Contaminated Soil
Ingénieur Conseil, JCLP HYPERBARIE - Paris
Abstract
The problem :
On the occasion of the boring of a sewage tube (diameter 3.5 m)
in Aubervilliers, close to Paris, an area which had been
contaminated with liquid kerosene had to be passed through for
about 300 metres. An extensive decontamination programme had been
completed; however some traces of free kerosene were suspected in
the ground and confirmed by soil sampling and analysis. The TBM
is a compressed air full face road header with a working pressure
of 1.9 105 Pa (0.9 b(g)). To cope with the
possible consequences from kerosene in the ground excavated by
the TBM, a risk evaluation was awarded to JCLP HYPERBARIE.
This risk
evaluation had to consider the three different situations:
1 - hyperbaric
interventions in the compressed air zone,
2 - possible
contamination of the tunnel atmosphere by kerosene from the
extracted ground,
3 - eventual
contamination of the environment via compressed air leaking
through surface ground.
Two types of
risks had to be evaluated : Fire / Explosion risks and toxicity
of potentially contaminated atmospheres.
Risk evaluation
: From the data of soil analysis kerosene could be evaluated
and the contaminated zones delineated. From kerosene vapour
partial pressures at the mean soil temperature, the possible
concentration of kerosene in the various atmospheres concerned
was calculated for comparison both with the explosive limits and
the toxic threshold values. Conclusions of the report
demonstrated that there was no risks of fire / explosions at any
of the places even when welding in compressed air; however the
same report showed that the toxic thresholds could be reached in
the worst case situations.
Safety precaution
: Specific safety rules have been established and implemented
: monitoring of kerosene concentration in the tunnel, in the
compressed air zone; information for the population concerning
possible odours in the boring TBM zone; mobilization of a mobile
team ready for environmental analysis of kerosene vapour.
Specifically for hyperbaric work, the known air working chamber
ventilation from leaks into the ground was estimated sufficient
to prevent any kerosene vapour to reach the toxic threshold
value. Therefore should hyperbaric manned intervention be
required during the crossing of the contaminated ground, then the
only specific safety rule would be kerosene partial pressure
monitoring with alarm in the compressed air environment.
Results :
Kerosene monitoring showed the expected contamination values in
the tunnel and in the compressed air zone. No odours where
detected in the environment nor were traces of kerosene. Routine
maintenance manned interventions in compressed air could have
been performed without restrictions during the excavation of the
contaminated ground.
During the
construction of a very large sewer network to collect all storm
water in the Paris area, several tunnels are being bored with
Tunnelling Boring Machines (TBM). One of these tunnels in the
town of Aubervilliers (3 km from Paris) had to cross through a
zone known to have been previously contaminated with hydrocarbons
which had leaked from various hydrocarbons storage tanks in an
old metal treatment factory.
Upon moving away
from this site that company had the legal duty to clean up the
underground. This was done by pumping out all liquid phase (5 tons
!). The major contaminant had been identified as kerosene.
Despite the underlying ground restoration operation some kerosene
was still adsorbed in the soil layer which was to be bored
through by the TBM.
An evaluation of
the risks associated with the presence of this contaminant has
been carried out in preparation for the passage of the TBM. It
was of major importance that the possible necessary actions could
be decided early enough not to stop the boring operation by
unexpected dangerous situations.
Levels of
hydrocarbons contained in the ground as determined by soil
investigation after the restoration and cleaning showed a maximum
concentration of total hydrocarbons of 3750 mg/kg of ground in
the most contaminated zone which has been controlled. The value
selected for calculations supporting the risk evaluation had been
4000 mg/kg. Those contaminants did not contain volatile
hydrocarbons, nor aromatics and could be classified as kerosene.
The TBM is a CSM
BESSAC machine. It is a road header machine fitted with a
bulkhead to form a compressed air chamber to support the ground
and hold back the water. The cutter boom operator is located at
atmospheric pressure and he can see the ground face and the
excavator machine through an acrylic view port. The excavated
ground is extracted from the cutting chamber, backward into the tunnel,
via a screw decompressing the ground directly into the muck skips
for removal.

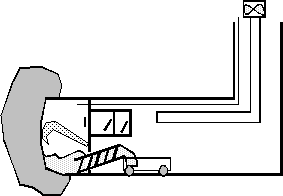
Fig 1 - Principle of CSM Bessac TBM Fig 2 - Hyperbaric cutting of an anchoring line
The compressed
air bubble is maintained at the pressure which is just sufficient
to remove all water from the cutting chamber. The selected
pressure is automatically adjusted by high flow regulators under
close monitoring from the cutter arm operator (figure 1). To
prevent large leaks of air from the cutting chamber into the
ground, a special foam is thrown onto the free face of the ground
with a directional foam jet. The foam is absorbed by the ground
and thanks to fast polymerisation reduces the ground gas
permeability.
The possible
dangerous consequences of boring through the contaminated soil
had to be evaluated in all areas which could be contaminated by
kerosene vapour. Risks eventually associated with the kerosene
vapour from the ground are related either to fire or toxicity,
both conditions need to be evaluated carefully.
The ground may
lead to evaporation of kerosene in two different places : in the
cutting chamber at pressure (mark A in figure 3) and in the
tunnel at atmospheric pressure during transportation of the
excavated material towards the shaft (mark B in figure 3). Both
situations need to be considered.
Some compressed
air is lost in the ground, although partly controlled by the foam
system. It will be released freely in the environment. Going
through the ground, this air will evaporate kerosene and may be
contaminated when it comes out of the ground at surface in the
adjacent streets (mark C on graph 3).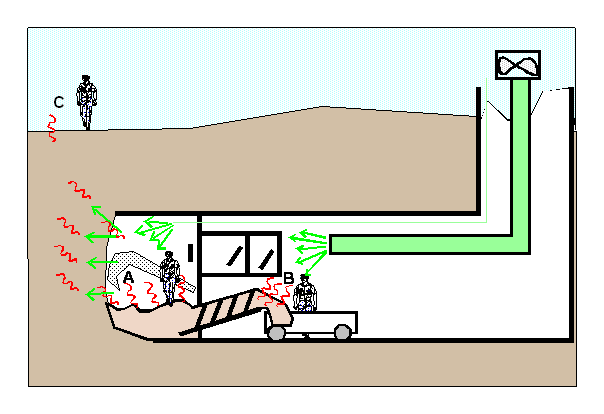
Figure 3 - Schematic of kerosene possible evaporation zones
| SOURCE |
NIOSH
[3] |
I
C S C [3] |
FFTS
[4] |
INRS
[5] |
MSDS
[6] |
| Hydrocarbon |
C9 to C16 | C10 to C18 | C9 to C16 | ||
| Exposure
limit |
100 mg/m3 | N
E |
5
mg/m3 (Oil mist) |
1500
mg/m3 |
N
E |
Vapour pressure
|
5
mmHg (37°C) |
>
8 mmHg (38
°C) |
1
mmHg (20°C) |
||
| Molecular
weight (mean) |
130 | > 145 | 170 | ||
| Vapour
density (air = 1) |
4.5 | > 5 | |||
| Flash
point |
37-65 °C | > 40 °C | > 38 °C | 38 °C | |
| Explosivity
limits |
0.7
– 5 % in air |
0.7
– 5 % in air |
1 – 6 % | 0.5 – 6 % | 0.7 – 5 % |
| Boiling
point (distillation) |
205-290 °C | 140-300 °C | 175-325 °C |
Table 4 -
Properties of kerosene as obtained from various sources
For the
appreciation of atmospheric contamination the most important data
is the pressure of saturated kerosene vapour at 20 ° C. This
vapour pressure, shown in table 4, will be the maximum partial
pressure of kerosene vapour at 20°C, when free evaporation takes
place in a confined space and when a steady state situation is
reached. This partial pressure will be the maximum pollutant
content of an atmosphere, even without any ventilation, at any
pressure at the temperature of reference.
The mean partial
pressure of kerosene vapour selected for the risk evaluation has
been 1 mmHg (MSDS value) or 1.33 hPa. Temperature in the ground
is close to 11 °C, therefore evaporation in the ground is much
lower than at 20°C, however for the risk evaluation a rough
estimate is acceptable provided the values are maximised. Since
the relationship between partial pressure and temperature was not
available, the 20°C value has been used in all cases (worst
possible situation).
Kerosene being a
mixture of various hydrocarbons from C9 to C16,
without any indications of the ratios for various components, the
mean molecular weight chosen for calculation is : M = 182 which
corresponds to the molecular weight of C13.
Properties of kerosene
- Toxicity :
The Threshold
Limit Value (TLV) selected for short exposure to kerosene vapour
in a breathable atmosphere has been 1500 mg/m3,
because INRS (Institut National de Recherche et de Sécurité)
produces a large body of evidence to support the published value.
This value must be calculated in partial pressure for use at any
pressure: it .can also be expressed in ppm (using the mean
Molecular weight 182 g, and a molecular volume of 24 litres for
20 °C) result is 197 ppm. This ppm value is a Fraction which
then needs to be translated into partial pressure (hPa). Since
this value is defined for 1 bar absolute, 197 ppm correspond to
197 10-6 bar or 0.197 hPa maximum kerosene partial
pressure (TLV in partial pressure – hPa-).
From the direct
comparison of the saturated kerosene vapour partial pressure
resulting from evaporation as described above (1.33 hPa) and the
TLV (0.197 hPa) it can be concluded that an atmosphere saturated
with kerosene at about 20°C would be beyond (7 times more) the
limit of acceptability at any pressure.
Evaporation
When an
atmosphere is in contact with the liquid phase of a volatile
organic compound (VOC), without ventilation, after a while the
level of VOC will reach its saturated vapour pressure value at
that temperature. Note: Kerosene is not a "VOC" because
the partial pressure at 20 °C is very low, however the vapour
behaviour model is equivalent).
Effect of ventilation
However when some
rate of ventilation exists, after a while a different steady
state level is reached. The resulting partial pressure of the
chemical is equal to the ratio of the off-gassing rate (l/min.)
of that chemical divided by the ventilation rate in the same
unit, provided that the ventilation is efficient and that
complete mixing takes place in the ventilated volume[1].
1 - In the
tunnel
Using the worst
possible case situation, it can be assumed that during
decompression of the ground in the screw, the small volume of air
included in the ground will be released into the tunnel and that
it could be saturated with kerosene vapour at the pressure of the
cutting chamber (between 0.5 and 1 bar(g) in this
case). Upon delivery in the tunnel this air is returned to
atmospheric pressure and the resulting partial pressures of
components are reduced by 1.5 or 2. It has been concluded that
the air delivered at the extremity of the screw at atmospheric
pressure cannot contain more that 1.33/1.5 hPa of kerosene or
0.88 hPa at atmospheric pressure. This small gas volume, even
with a minimal dilution by the ventilation of the tunnel will not
lead to any risk of toxicity for the workers in the tunnel (a few
litres/sec against at least 1 m3/sec).
2 – In
the cutting chamber
Should a need for
a hyperbaric inspection or repair arise during the boring in the
contaminated soil, the partial pressure of kerosene in the
cutting chamber atmosphere is reduced as explained above by the
high rate of ventilation of the cutting chamber to match the air
leaks in the ground through the face. In addition, before
excavation, traces of kerosene remaining in the ground are
ventilated away by air leaks in situ. The mean flow
considered for the risk evaluation is 60 m3/min. To
reach a toxic partial pressure of kerosene in the cutting chamber
it would be necessary to evaporate 0.118 m3/min of
pure vapour of kerosene…or 118 x 182/24 = 89.5 g of kerosene
per minute…. This was estimated as very unrealistic when one
takes into account the possibility of kerosene adsorbed onto the
bulk ground to evaporate into the ambient atmosphere only from
the free surface of ground sitting in the cutting chamber (mark B
on figure 3). It was concluded that kerosene cannot be a toxic
risk for compressed air workers in that situation.
3 – In
the external environment
Air escaping from
the ground in the environment can be assumed to be saturated with
kerosene at a pressure close to atmospheric pressure (worst
possible case) potential toxicity reduction effect of
decompression is not taken into consideration because very little
information is available concerning the exact pressure of
evaporation…. In that case air escaping freely from the
ground will contain anyhow a maximum partial pressure of kerosene
7 times more than the TLV.
When that air is
reaching the surface in open air, immediate dilution will reduce
the toxicity far below the TLV.
When that air is
released in a semi-confined zone, like cellars below buildings it
may accumulate and create a “toxic zone” until dilution
will reduce the potential danger.
In addition the
odour threshold of kerosene is below the TLV. Which means that it
can be detected by human nose at partial pressures lower than
TLV.
Some action must
be taken to protect population in non ventilated areas and to
make sure every one concerned is informed that some smell may be
noticed, however it would not be dangerous for their health,
however it must be properly reported.
4 – Spoil
disposal
The excavated
ground from this zone is considered contaminated and is dumped
under controlled conditions in a specially selected discharge
plant.
Properties of
kerosene - Fire :
Temperature of
self-inflammation of kerosene in air is very high : above 220 °C
and Flash point in air is above 38 °C
This means that
an atmosphere saturated at 20°C with kerosene cannot flash
spontaneously even with a spark.
The Lower Limit
of Explosivity is 0.5 to 0.7 % corresponds to 5000 to 7000 ppm at
atmospheric pressure. We have shown that at 20 °C this
concentration would be higher than the kerosene saturated vapour
pressure (1.33 hPa) : kerosene cannot stay in gas phase at
concentration of 5000 ppm at 20°C, it will either not evaporate
enough or would condense.
In the tunnel and
in the environment, conditions of Lower Explosive Limit can never
be attained.
There
is no risk of fire even with open flames
While working
under pressure one should consider the effect of the increased
partial pressure of oxygen on flammability of hydrocarbons. It
has been clearly established by Cleuet et al. (1994) [2] that in
the range of pressure concerned (0 to 10 bar(g)), in
compressed air, the explosive limits of hydrocarbons (expressed
in Fraction -ppm or %-) are not significantly modified from those
measured at atmospheric pressure.
Therefore is can
be considered that also in compressed air, in the conditions of
tunnelling described, there is no risk of explosion even in case
of open flame exposure.
In order to cope
with any unexpected condition like a high concentration of
kerosene in the ground which would have gone undetected during
the ground control campaign or an insufficient dilution in the
cutting chamber while hyperbaric work would be in progress…
several extra safety precautions have been implemented before
crossing through the suspected zone.
Kerosene vapour analysis :
Controls for
kerosene contamination levels have been organised with two
independent systems:
Samples of the
extracted ground were collected in the shaft, at regular intervals,
each sample was quickly controlled on total hydrocarbons content
to monitor the penetration of the TBM into the contaminated area.
Gas sampling with
continuous monitoring (DRAEGER type Polytron IR Ex) calibrated
with air saturated with kerosene at the temperature of the tunnel
(1.33 hPa or close to) with an audible and visual alarm point set
at 30 % of this saturated value.
There were 2
sampling lines, manually selected: one sample could be pumped
from near by the delivery point of the screw, the other one was
drawn from the working chamber to monitor the atmosphere in case
of manned hyperbaric intervention.
An information
bulletin was distributed to the near-by population and displayed
in the buildings around the zone to be excavated by the TBM. This
bulletin explained how some kerosene vapour could be smelled when
the machine will pass below but that there is no specific danger.
A phone number for help was also indicated in case of smell in
confined areas.
Information was
also discussed with the fire brigade in a special meeting where
all details of the study were explained (and accepted).
All tunnel
workers have been informed on risk evaluation and safety
measures. The team leader of each shift underwent the necessary
training concerning the gas monitoring system and actions to be
taken in case the alarm would be triggered.
A portable total
hydrocarbon analyser calibrated in the same manner was made
available to a mobile team, ready to go on sites in case an
information would be received from the population. A small number
of exposed cellars were selected for actual controls during the
passage of the machine.
When the machine
reached the contaminated zone soil analysis confirmed the
presence of kerosene and odour of kerosene was acknowledged in
the tunnel. The gas detector showed not more than 1/3 of the
saturated maximum value on the gas close to the decompressed
ground.
No neighbour ever
complained or called the special phone number, no traces of
kerosene vapour were ever detected in the streets or cellars.
Crossing the zone
(60 m) lasted 5 days.
Few routine
hyperbaric interventions for maintenance of the cutter arm were
carried out, no personnel have reported kerosene odour during
those interventions. Should a repair requiring welding or arc-air
burning had become necessary, it would have been carried based on
the evidence that there was no risk of fire whatsoever.
Facing a
situation which had not been met in previous excavations with Bessac
type TBMs, it was decided to thoroughly evaluate the risks, in
the worst possible conditions. Based on the information obtained
from cleaning campaigns and on the pollutant physico-chemical
data it was possible to demonstrate that the risks of toxicity
were minimal and that the risk fire was non-existent.
For extra safety
precautions several control systems were implemented on site and
in the tunnel which confirmed that the evaporation of kerosene
was very mild and has never reached values of concern.
This approach of
atmospheric contamination by vapours, based on maximum partial
pressure of saturated vapour can be used for organic compounds
even when their volatility is very low.
Incidentally, the
day following the relieving of safety precautions in relation
with kerosene, a burst of Hydrogen Sulphide (H2S) was
detected (from smell by the operator) which led to tunnel
evacuation until an extra ventilation system could be installed
(3 hours). The contaminated ground zone was probably only a few
meters long. All detectable hydrogen sulphide was gone within a
few hours of further boring.
The training and
preparation of the crew to cope with an expected atmospheric
contamination in the tunnel proved to have been useful since an
early alert was given for an unexpected one.
References :
1) CHOUTEAU J.,
BIANCO V., ORIOL P. et all., Expérimentation animale et humaine
de vie prolongée sous pression en atmosphère oxygène-hélium.
Technologie et résultats biologiques. 1967, Annales de
l'Anesthésiologie Française, T VIII, nE spécial 1, 1-45.
2) CLEUET A. and
P. GROS (Mise à jour 1994 par J. M PETIT) – 1994 Les
mélanges explosifs, Gaz, Vapeurs, Poussières, Liquides, Solides
– INRS éditeur – ED 335. Paris
3) NIOSH : Pocket
guide to chemical hazards. Translated by International Safety
Card. Programme International sur la sécurité des substances
chimiques – Commission Européenne 1993
4) FTSS –
Centre Canadien d'hygiène et de sécurité au travail. Fiche
FTSS 1094432 – Kerosene – 2000-3
5) INRS Fiche
toxicologique n° 140 Pétroles Lampants. INRS 40 rue Noyer 75014
– Paris FT 140 pp 4 – 1996
6) MSDS –
material safety data sheet : http://www.jtbaker.com:80/mdss/k2175.ht
from Mallinckrodt Baker, Inc. 222 Red School Lane, Phillipsburg,
NJ 08865 USA.
Acknowledgement : SIAAP and Montcocol SA
have authorised the publication of those results for benefit of
the tunnelling community.